Northern District of California enacted its patent rules in January 2001. The district is now ready to reevaluate those rules and has asked for public comments and concerns. Ed Reines, who is on the N.D. Patent Local Rules Committee has asked whether Patently-O readers have some input on potential improvements to the rules:
The committee knows that the patent community has a variety of views on patent local rules. Because a healthy debate and full ventilation of issues is likely to lead the best outcome, the committee seeks broad input on potential changes to the patent local rules.
The Northern District of California patent rules have served as a set of model for a number of districts contemplating their own patent specific local rules. In addition, many district judges across the country require litigants to follow some form of the rules. Comments should be e-mailed to patentlocalrulescommittee@gmail.com and submitted by December 15, 2006.
My initial comments: (1) We need to revisit claim construction proposals and get the court involved with the technology a bit earlier. As we have seen in several CAFC cases, the claim construction process requires a very good understanding of both the invention and the accused infringing device. The CAFC has also now clarified the roles of various types of evidence used in claim construction. Claim construction proposals should include clear tables showing how the various levels of evidence support the proposed claim construction. (2) In addition, the court should set-up rules to ensure that there is an early understanding of whether the plaintiff has a chance at obtaining injunctive relief. This will help guide settlement.
 PHG Tech v. St. John (Fed. Cir. 2006).
PHG Tech v. St. John (Fed. Cir. 2006). 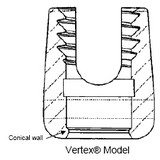 Medtronic won summary judgment of non-infringement for its Vertex spinal implants, but the jury found that its MAS spinal screws were infringing. Both sides appealed, and we discuss two issues here:
Medtronic won summary judgment of non-infringement for its Vertex spinal implants, but the jury found that its MAS spinal screws were infringing. Both sides appealed, and we discuss two issues here: 
 Abraxis (AstraZeneca) v. Mayne (Fed. Cir. 2006).
Abraxis (AstraZeneca) v. Mayne (Fed. Cir. 2006). 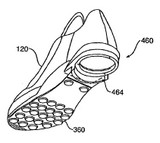 Akeva v. Adidas (Fed. Cir. 2006, NON-PRECEDENTIAL)
Akeva v. Adidas (Fed. Cir. 2006, NON-PRECEDENTIAL) By
By 


 Abbott Labs. v. Baxter Pharm. (Fed. Cir. 2006).
Abbott Labs. v. Baxter Pharm. (Fed. Cir. 2006). From IP Law360
From IP Law360
 In response, Alderucci notes that the Examiner “has completely misinterpreted the most popular and analyzed book in all of human history. . . . [Nevertheless], the claims as pending have been carefully amended to steer clear of embracing of any of ancient practices of Joseph, the Pharoah, or the [ancient] Egyptians.”
In response, Alderucci notes that the Examiner “has completely misinterpreted the most popular and analyzed book in all of human history. . . . [Nevertheless], the claims as pending have been carefully amended to steer clear of embracing of any of ancient practices of Joseph, the Pharoah, or the [ancient] Egyptians.”  Grocer Meijer has reportedly filed an antitrust suit against drug maker Eisai for monopolization of the U.S. market for its billion-dollar GERD treatment Aciphex. (S.D.N.Y.). Meijer charges that the patent was obtained fraudulently and that it is either invalid or unenforceable. Meijer runs pharmacies in its stores, and the patent threat keeps generics out and prices high.
Grocer Meijer has reportedly filed an antitrust suit against drug maker Eisai for monopolization of the U.S. market for its billion-dollar GERD treatment Aciphex. (S.D.N.Y.). Meijer charges that the patent was obtained fraudulently and that it is either invalid or unenforceable. Meijer runs pharmacies in its stores, and the patent threat keeps generics out and prices high.